11 Aug 23
This article will help you get a better understanding of the rules and regulatory bodies that surround medical device marketing in the UK and EU.
At the end of this article, you should be able to explain what intended purpose is and how it affects your marketing, outline the main principles of visual medical device regulations, and know how to prevent any regulatory repercussions.
Medical device imagery is any visual aid you use in your marketing. It can come in a variety of different forms, from line drawings, logos, graphics, videos, animations and photography. Anything that is seen by your audience counts as imagery.
While these visual elements can add huge value to your campaign, there are rules surrounding the types of images that you can use, all coming back to your intended purpose.
Intended purpose refers to your device’s primary function and explains what the device does, who it treats, its clinical conditions, where it’s used and the clinical claims that your company wants to make in its marketing materials about the device. These clinical claims can be clinical, economic or technical amongst other things.
When registering your device with your regulation body (also known as competent authority) such as the MHRA (Medicines and Healthcare Regulatory Agency, UK) or the EU MDR (European Medical Device Regulations, Europe) you must submit your intended purpose to them for approval.
Knowing and fully understanding the intended purpose of the device you’re marketing is critical to ensure that you adhere to each country’s medical device marketing regulations. However, when it comes to Europe you not only have to comply with the EU MDR but also take note of the local country regulations which are often much more stringent as to who and how you can market your medical device.
The imagery you’re allowed to use is impacted by the information included in your intended purpose.
This means that if your clinical data approved by your regulatory body was only made up of female patients aged between 50-60, you can’t depict anyone in your marketing who doesn’t match this demographic.
Another example could be a device related to patient weight loss, if your device shows an average loss of 50% of excess weight, you cannot show any imagery that suggests a greater weight loss could be possible. All your imagery must reflect the data approved for your intended purpose.
We’ve created a list of categories that you should keep in mind.
The intended purpose itself is just one (very important) aspect of medical device regulations, in fact, the rules you need to be aware of fall into three categories.
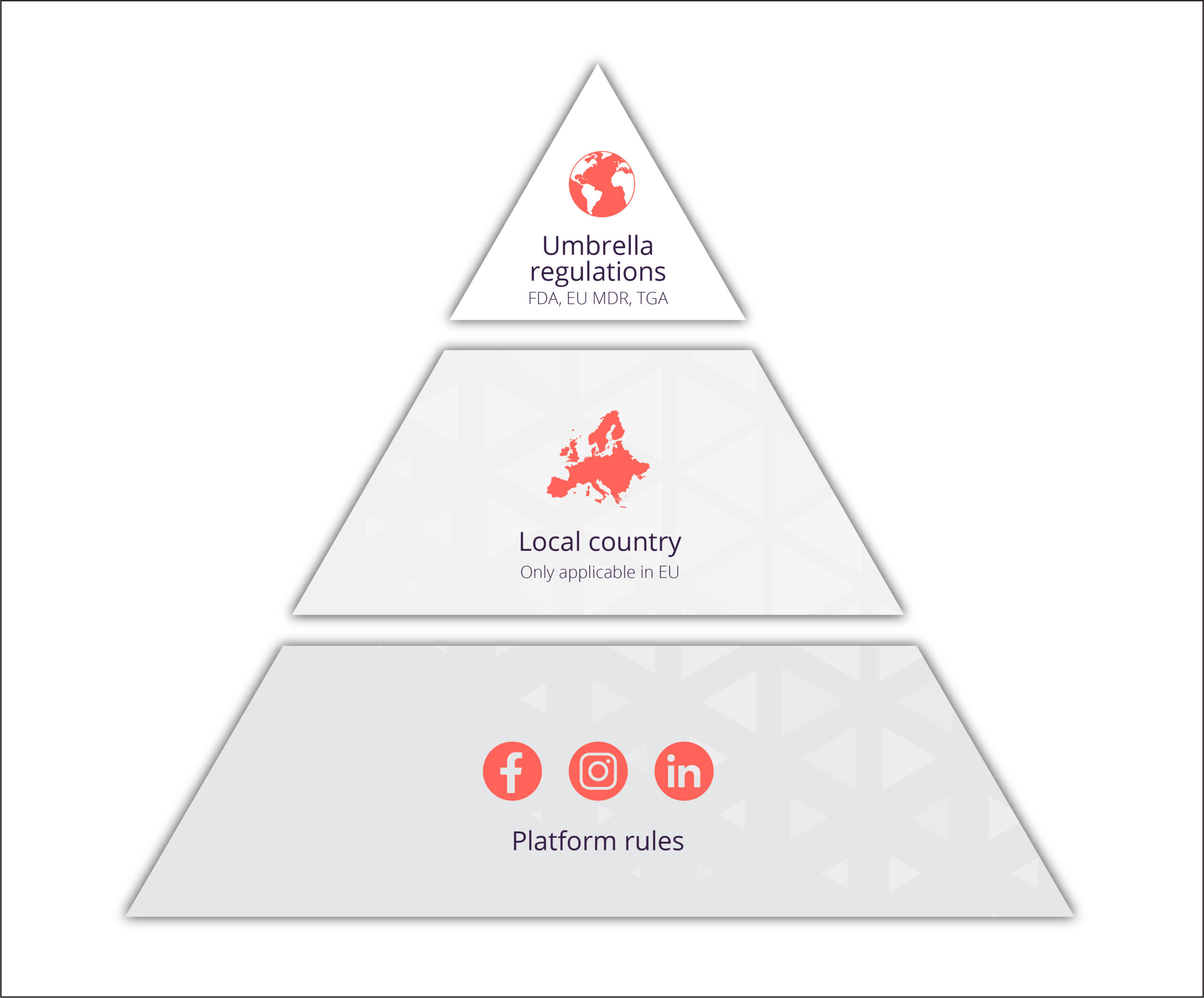
Think of it like a three-tier system. At the top, you have umbrella regulations which are governed by the regulatory bodies, such as the EU MDR or MHRA, which covers the whole of the EU and UK respectively.
In the second tier, you have the local country regulations which come from individual countries. These only apply to the EU as it is made up of individual member states, each with their own rules on the promotion of medical devices. To find our more about each countries regulations read our guide on UK and EU MDR advertising guidelines.
Finally, at the bottom, there are the platform rules which are set and determined by the social media channel you’re posting on.
As discussed earlier, you need to submit your intended purpose (or equivalent for the country you are entering) to the appropriate regulatory body every time you enter a new geography (for instance EU MDR, FDA, MHRA, TGA). This poses the question: what happens if audiences outside of one of your approved geographies view your marketing?
Here, social media transcends borders. If you aren’t marketing directly to an audience outside your approved geographies, there isn’t a problem as long as it’s clear your marketing was not intended for that audience (e.g. USA – if there’s a risk American citizens will see a post, it must say on it ‘Not for Sale in the United States of America’ so there can be no confusion).
This is more straightforward in Europe as a clear distinction can be made by the language the post is written in.
A good rule of thumb is to always adhere to the regulations that are most stringent in the markets you operate.
While most countries have somewhat similar rules, this article will be focusing on the general UK and EU regulatory rules from both the EU regulatory bodies and social media platforms.
Fair and accurate representation is a pillar in almost all guidelines set out by regulatory bodies. The images you use can’t be misleading, biased, or untruthful in any way. This ties back to the importance of the intended purpose, so you can’t visually imply your device can do anything that isn’t in your intended purpose.
Another rule is the importance of consent and the dignity of the subject. This is particularly called out on many social media platforms.
You must have the consent of the subject when it comes to how you use their image and how you depict it. It isn’t just about what you can say it’s also about how the person contained in the image will feel.
So long as you act in a proper manner to all concerned, the viewer and the person in the image, you should adhere to these rules. This mainly refers to photographic and video content which depicts people.
As mentioned previously in the article, platforms make up the third tier of the rule’s marketers need to follow when creating content, this section of the article will briefly explore the guidelines suggested by the most popular platforms.
Meta guidelines (Facebook and Instagram)
Meta’s guidelines prohibit any kind of content that promotes misinformation, harmful self-perception, and injury.
This authenticity and accurate representation are at the forefront of these guidelines. It’s against guidelines to produce content that fosters misinformation and negative or unhealthy body images. Furthermore, graphic images, for example, surgery, may count as images of injury, so maybe flagged (especially if you are running ads).
LinkedIn guidelines
LinkedIn guidelines say that medical device content can only be targeted towards HCP (Healthcare professionals) and not a wider consumer or general audience.
They also require respect and dignity in your content for both the viewer and the subject, not depicting injury or anything that the general audience may find upsetting or disturbing.
Twitter guidelines
Twitter has a long list of healthcare guidelines that are designed to protect its users and their online space. In addition, they have different guidelines per country, so we suggest that you follow the link and stay informed about the guidelines that affect your region.
Now that you have a better understanding of the rules you must adhere to, you might be wondering how to put this all into practice and steer clear of trouble.
If you’re nervous about what images or graphics you can use, speak to your internal regulatory department. They’re the ones in your company that are in charge of what you can and can’t show in your marketing.
It may be good to present a whole range of images and get them approved in one go, that way you’ll have a large bank of images to call on when you’re pulling your marketing campaign together, rather than having to get approval every time you want to use an image.
Like we said before, your intended purpose has all the information you need when coming up with your marketing imagery. Focus on what it does say, not what you want it to say and cater your marketing from there.
Hopefully, this has made your life a little bit easier and answered all the questions that had been buzzing around your mind. It can be difficult to know what you can and can’t show in medical device, but so long as you always refer to your intended purpose, you’re unlikely to go too far wrong.
Similar articles that you might find interesting.
Are you a marketer struggling to understand how regulations affect your role? Are you in need of a clear, concise guide that tells you exactly what you can and can’t show in your marketing campaigns?
We use cookies to give you the best online experience. By agreeing you accept the use of cookies in accordance with our cookie policy.
When you visit any web site, it may store or retrieve information on your browser, mostly in the form of cookies. Control your personal Cookie Services here.