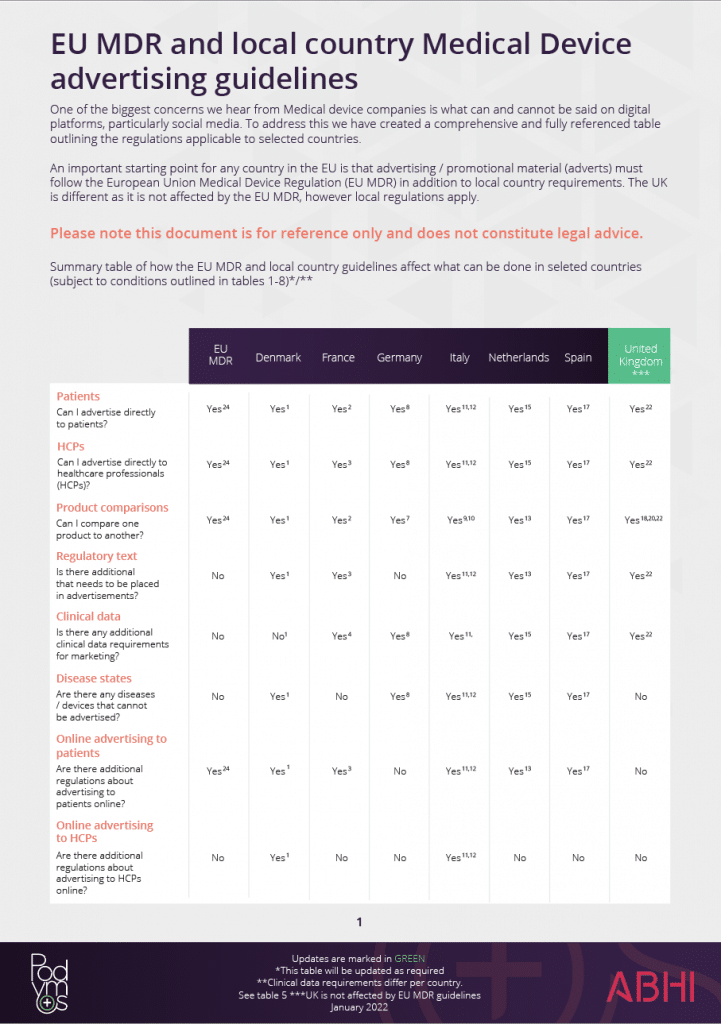
The EU MDR doesn’t address online marketing requirements but does require that Medical Device websites be seen as labels. As such, the rules for traditional marketing also apply to social media and digital marketing.
In summary, this means that so long as your post, article, or content aligns with your intended purpose, is supported by the correct clinical data, and your content is not misleading in any posts, images or trademarks you will comply with the EU MDR.
In specific Member States, you will need to meet local advertising and promotion requirements for all online advertisements: for example, in the Netherlands, as in many other territories, you will need to consider language requirements. As the internet is available to everyone, online advertisements will need to be aligned with patient marketing requirements.
This is not always the case however, as in France and Denmark, Healthcare Professional marketing can only be undertaken if the specific sections are password-protected, whereas in Italy, it same information can be provided online with disclaimers.